This medication requires a valid prescription. Please refer to the ‘Prescription Guide’ for details.
Note: We can assist only if the medicine is unavailable in your country.”
MitoGENE supports patients and doctors in India by assisting with:
- Documentation and regulatory filings
- Import coordination for Mektovi (binimetinib)
- Secure delivery compliant with Indian healthcare regulations
This ensures a safe and legal pathway for Indian patients seeking international cancer medicines like Mektovi (binimetinib) when no local treatment alternatives are available.
Need Help Accessing Mektovi (binimetinib) in India?
If you or your loved one has been prescribed Mektovi by an oncologist, MitoGENE can support you with:
- Fast-track documentation
- International sourcing and delivery
- Expert NPP compliance
Reach out to us via the inquiry form below.
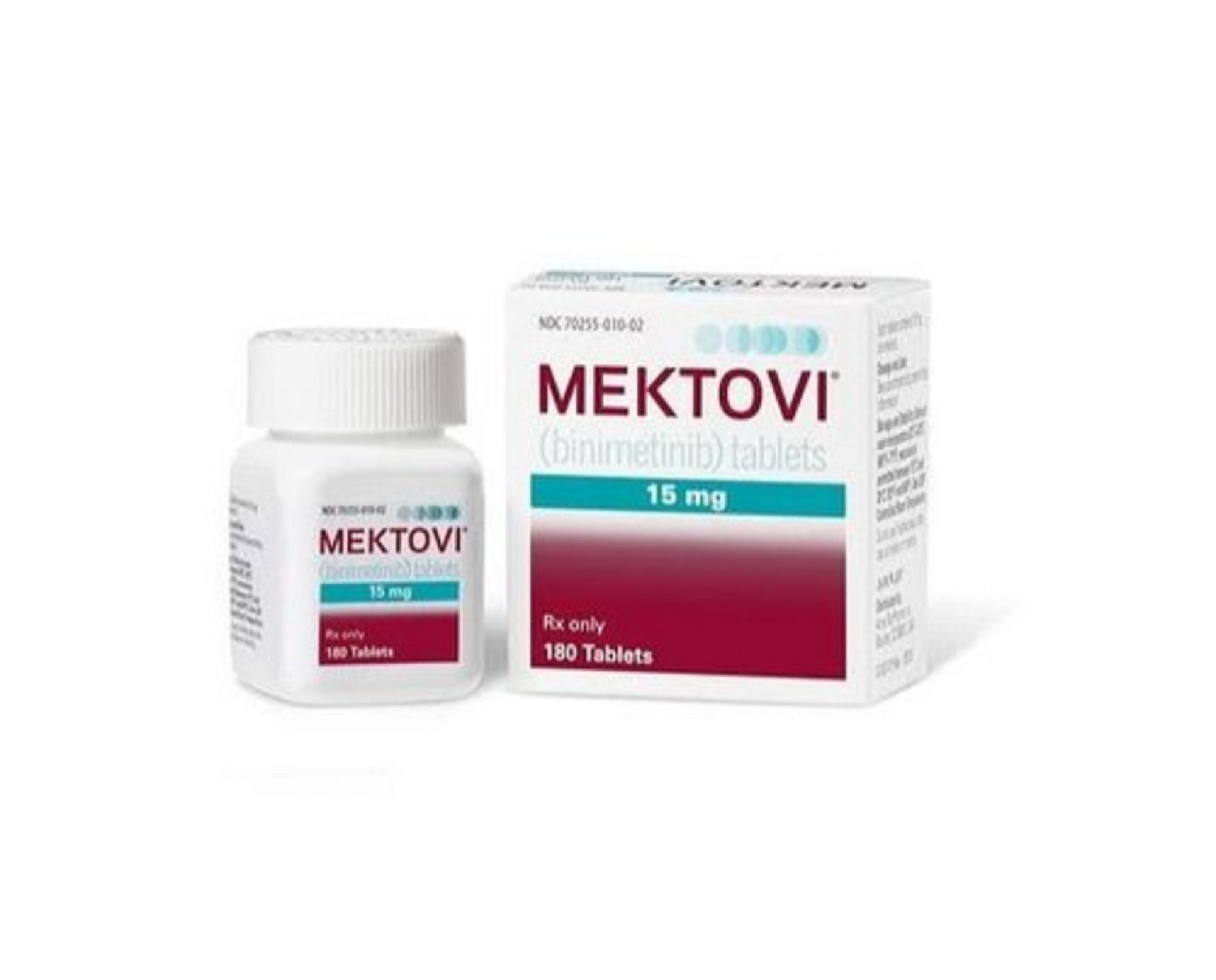
MEKTOVI (binimetinib)
Mektovi (binimetinib) is a targeted cancer therapy used in combination with Braftovi (encorafenib) to treat adults with unresectable or metastatic melanoma that has a BRAF V600E or V600K mutation, as detected by an FDA-approved test. Mektovi is a MEK inhibitor that works by interfering with the MAPK/ERK signaling pathway, which plays a key role in the growth of certain cancers.
Access in India (Named Patient Program)
Mektovi (binimetinib) is not commercially available in India. However, patients may be able to access this medicine through the Named Patient Program (NPP), a legal framework that allows Indian patients to import medications not yet approved domestically, under the supervision of a licensed treating physician.
Medicine Approved by
- United States (U.S.) – U.S. FDA
- European Union (EU) – EMA
- Australia – TGA
- Canada – Health Canada
Disease Indications: Skin Cancer
Disease Indications: Skin Cancer
Manufacturer: Pierre Fabre Medicament
Usage: Oral
Available Dosage Form & Package:
- 84 tablets of 15 mg
- 168 tablets of 15 mg
Shipping:
Room Temperature Shipping. Mektovi (binimetinib) is shipped under controlled room temperature conditions (15°C to 25°C) using standard logistics procedures.trolled room temperature range of 15°C to 25°C throughout transit.