This medication requires a valid prescription. Please refer to the ‘Prescription Guide’ for details.
Note: We can assist only if the medicine is unavailable in your country.”
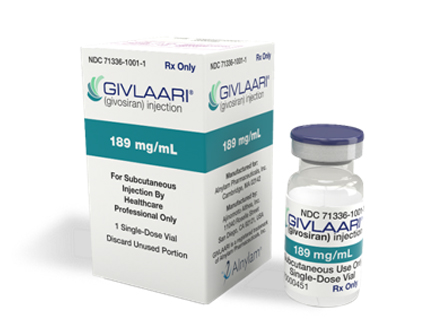
Givlaari (givosiran)
General Description
Givlaari (givosiran) is prescribed for the treatment of acute hepatic porphyria in adults and adolescents aged 12 years and above.
Getting Givlaari (givosiran) in India
Givlaari (givosiran) is approved in the U.S., Europe, and other countries, but it is not yet commercially available in India. However, eligible patients in India may access this important therapy through the Named Patient Program (NPP).
MitoGENE helps patients obtain Givlaari (givosiran) legally and safely, in collaboration with their treating doctor. We assist with documentation, import coordination, and delivery—ensuring full compliance with Indian regulatory guidelines.
If you or a loved one needs Givlaari (givosiran),MitoGENE is here to guide you through every step of the process.
Disease Indications:Acute Hepatic Porphyria (AHP)
Manufacturer:Viela Bio
Usage:Subcutaneous
Medicine Approved by:
- U.S. Food and Drug Administration (FDA)
- European Medical Agency (EMA)
- Pharmaceuticals and Medical Devices Agency (PMDA)
Available Dosage Form & Package:
- Single dose vials 189mg/ml
Shipping:Cold Chain Shipping. Certain medicines can be affected by heat, light, or improper handling. Cold chain shipping uses temperature-controlled packaging and transport provided by specialized medical couriers to keep these medicines stable and effective. Due to the added care and equipment involved, this method is often more expensive than standard shipping.