This medication requires a valid prescription. Please refer to the ‘Prescription Guide’ for details.
Note: We can assist only if the medicine is unavailable in your country.”
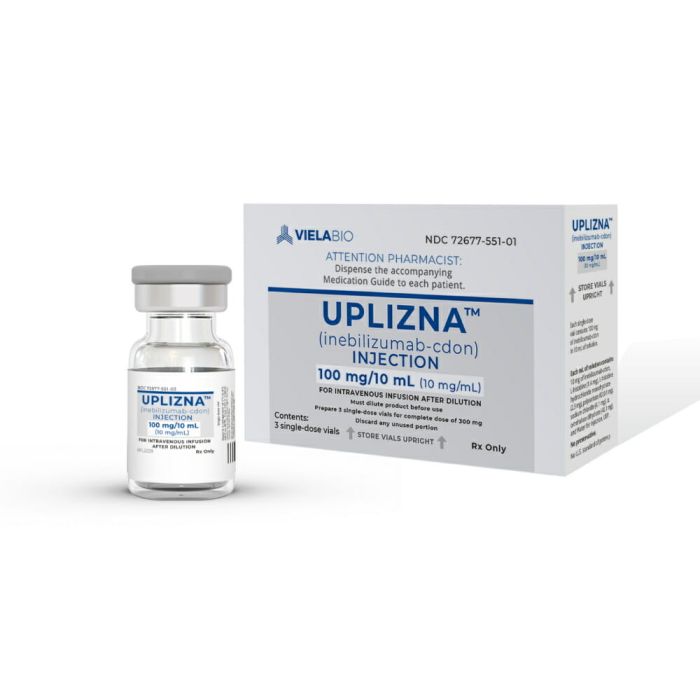
Uplizna (inebilizumab-cdon)
General Description:
Uplizna (inebilizumab-cdon) is a monoclonal antibody medication used for the treatment of adults with neuromyelitis optica spectrum disorder (NMOSD) who are anti-aquaporin-4 (AQP4) antibody positive.
It works by targeting and depleting CD19-positive B cells, a type of immune cell that plays a central role in NMOSD pathogenesis. By reducing these cells, Uplizna helps prevent relapses and inflammation in the optic nerves and spinal cord.
Uplizna is notable for being the first approved NMOSD therapy administered twice yearly, offering patients a long-acting and convenient treatment option.
Disease Indications:
Neuromyelitis Optica Spectrum Disorder (NMOSD)
Manufacturer:
Viela Bio
Usage:
Intravenous
Medicine Approved by:
• European Medicines Agency (EMA)
• Food and Drug Administration (FDA)
Package:
• 3 single-dose vials × 100 mg/10 mL
Shipping:
Cold Chain Shipping
Uplizna contains temperature-sensitive biological ingredients that may degrade when exposed to heat, light, or improper handling. Therefore, it is transported using cold chain delivery, which ensures controlled temperature conditions through specialized couriers, insulated packaging, and temperature-regulated vehicles.
Although cold chain shipping may be more expensive than standard delivery, it guarantees that Uplizna arrives safe, uncompromised, and fully effective for clinical use.